Abstract
Patients with severe aplastic anemia (SAA) are at high risk for infections due to prolonged neutropenia. Results of a historic multicenter randomized control trial of granulocyte transfusions (GTs) in patients with severe neutropenia failed to demonstrate significant differences in the composite outcome of overall survival (OS) and microbial response but was limited by patient accrual and variable and often low granulocyte collections and dosing. In the current study, we aimed to characterize the role of GTs in SAA as they relate to clinical response, OS, and as a bridge to curative therapy with hematopoietic stem cell transplant (HSCT).
SAA patients treated with IST who received GTs at the National Institutes of Health between 2011 and 2020 were included. Primary outcomes were survival from first GT, including OS, survival to discharge, and survival to HSCT conditioning. Secondary outcomes included evaluation of response at 7 and 30 days after initiation of GTs based on clinical, microbiological, and radiographic criteria. Cox proportional-hazards model was used to determine impact of neutrophil response and infection type on survival. Kaplan-Meier estimators were used to assess OS.
Twenty-eight SAA patients with median age 20 years (range 6-65 years) underwent 30 GT courses administered prior to (n=16), during (n=8), or after HSCT (n=6). Initial IST included horse antithymocyte globulin (hATG), cyclosporine (CSA), and Eltrombopag (n=11), hATG/CSA (n=8), cyclophosphamide/CSA (n=6), and hATG/CSA, and sirolimus (n=1). Two patients did not receive any IST and went directly to HSCT. A median of 8 granulocyte products per course (range 1-39 products) were administered over a median of 23.5 days (range 3-103 days), with a per-dose median of 1.28 x 10^9 granulocyte cells/kg (range 0.45-4.52 x 10^9).
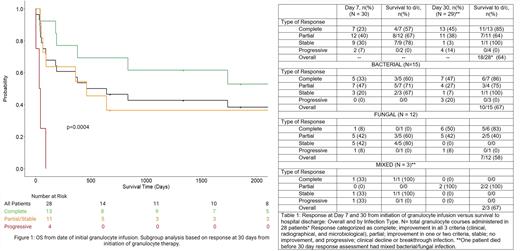
Indications for GT included invasive bacterial (n=15), fungal (n=12), and mixed (n=3) infections. Survival to discharge in GT course recipients who achieved complete response by day 7 or day 30 increased from 57% (4/7) to 85% (11/13), respectively. OS from initial GT was 50% at median length of follow up at 550.5 days (range 2-4213 days). Patients who achieved complete, partial, or stable response at 30 days had significantly improved OS compared to non-responders (p=0.0004) (Figure 1). Sixty-four percent (18/28) of patients survived to hospital discharge (Table 1). Sex, type of infection, or % of days with ANC >200 cells/mcL during the granulocyte course, were not predictive of survival (p=0.52, p=0.7, p=0.28 respectively).
Eighteen of 21 (86%) patients awaiting HSCT during granulocyte therapy ultimately underwent transplant, and 62% (13/21) of them survived to discharge. Of three patients awaiting HSCT who did not receive HSCT, two died and one had hematopoietic recovery after IST and survived. Five patients who received HSCT but did not survive to discharge died due to progression of initial infection (n=3), graft-versus-host-disease (n=1), and secondary hemophagocytic lymphohistiocytosis (n=1).
GTs at high doses can be a valuable adjunctive therapy to manage severe infections in SAA and may impact survival in those with improvement or stabilization of their underlying infection. OS in this population remains poor, but GTs may be a useful tool to bridge patients to curative treatment with HSCT.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal